While the first cases of COVID-19 in Wuhan occurred at a live animal market, person-to-person transmission soon became the norm of spread. Transmission is via respiratory droplets in a manner similar to influenza; the droplets do not travel more than 6 feet and do not travel in the air, hence the recommendation for social distancing. Each person with COVID-19 will infect on average 2.2 others (for influenza, that same figure is 1.3) There has been a lot of changes in the recommendations for precautions to be used in the care of patients with COVID-19. Initially, given the uncertainty surrounding transmission, airborne precautions were used. Virtually all guidelines now recognize that droplet precautions are adequate for most clinical situations unless there is a high risk of aerosolization. While one study from NEJM from March 17th revealed that viral particles remained viable in an aerosolized form in experimental conditions for close to three hours, there has no been definitive evidence connecting that fact to actual infections.
Bottom Line: Droplet precautions provide adequate protection to health care workers taking care of COVID-19 patients.
The best data on the clinical characteristics and diagnostic strategy of COVID-19 comes from the original Chinese cohort as reported in February in this NEJM publication.
This retrospective study analyzed over 1000 patients who fell ill with COVID-19 in the very early stages of the epidemic. The researchers discovered that a dry cough and fever were the most common clinical signs at presentation and that nasal congestion and abdominal complaints were fairly rare. The median incubation period was 4 days. CXR and chest CT were abnormal in the majority of cases, revealing bilateral ground glass opacities. On laboratory evaluation, lymphopenia was the most cardinal feature noted. In addition, non-specific markers of inflammation such as CRP, D-Dimer, LDH were often elevated.
Bottom Line: Consider COVID-19 in any patient presenting with fever and cough. Lymphopenia, bilateral infiltrates and elevated inflammatory markers would support the diagnosis.
No specific clinical trials have been performed to show that a specific treatment is beneficial in COVID-19. Several medications are currently being used.
Steroids
- These have remained controversial. The largest study to date, in JAMA Internal Medicine from March 13th, looked at steroids for severe COVID-19 found that they might be possibly helpful. However, this contrasts with the following data:
- Steroids were routinely used with SARS in 2002 (to presumably reduce inflammation) and led to no benefit and caused harm
- Steroids are associated with delayed viral clearance in patients with MERS and are not used in that coronavirus disease
- Steroids lead to increased mortality in influenza patients
- Given all of the above, steroids are currently NOT recommended in the treatment of COVID-19 patients unless there is another compelling indication such as a COPD flare.
Hydroxychloroquine
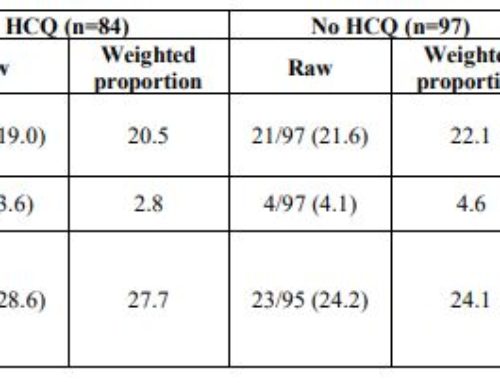
- Known from basic science to inhibit coronavirus replication by increasing endosomal pH and blocking glycosylation of the SARS-CoV-2 receptor. In addition, achieves excellent tissue penetration into the lungs. A study from China looked at the in vitro activity of hydroxychloroquine (Plaquenil) and found it to be effective in reducing viral replication. Based on kinetic modeling from that study, the following dose is used:
- Loading dose of hydroxychloroquine 400 mg po BID for 2 doses
- Hydroxychloroquine 400 mg daily for 4 more days
- Finally, this medication’s well-established side effect profile has reassured clinicians that while its benefit is unproven, there is almost certainly no harm. Additionally, azithromycin may be added to hydroxychloroquine for its enhanced anti-inflammatory effects.
Antivirals
- The antiviral medication remdesevir holds the greatest promise so far. This medicine, initially developed for Ebola and showing great activity against COVID-19 in vitro, is now being studied for both moderate and severe COVID-19 disease. Consider enrolling your patients in a clinical trial if one is available at your institution. Alternatively, consider compassionate use of remdesevir if the medication is available for such a purpose.
Bottom Line: Avoid steroids, consider hydroxychloroquine and possibly add azithromycin
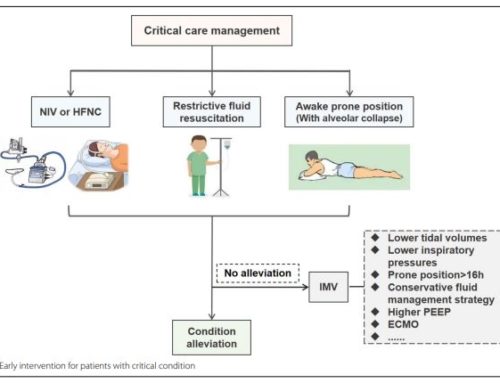
The clinical outcomes of COVID-19 will probably vary from country to country. In the earliest studies from China (JAMA February 7th and JAMA Internal Medicine on March 13th), several things became apparent. Close to 25% of the patients admitted to a hospital required ICU level of care with ARDS being the most feared complication. The mortality of COVID-19 has varied in most countries but is probably around 2-5%. Risk factors for the development of ARDS include advanced age and neutrophilia (possibly related to cytokine storm), as well as elevated D-Dimer over 1000, elevated LDH over 245, and elevated ferritin. Early data for similar patients in the United States have confirmed the above findings with the addition of high rates of myocarditis noted in COVID-19 patients. Additionally, obesity has been recently noted as a possible risk factor for severe COVID-19 disease.

What a great summary. Thanks, Steven. An ER doc I know, got a UV light cleaner for his phone and as soon as he gets home takes a shower with his eyeglasses and watch on. You are a hero! Love you and sending you good vibes and prayers every day.