They Key to Avoid Unintended Consequences of Health Care Personnel Vaccination During a COVID Surge
Background
Following the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee’s 17 to 4 vote in support of the FDA granting Emergency Use Authorization (EUA) to the Pfizer/BioNTech mRNA vaccine, the vaccine is being shipped to states for administration to health care personnel and long term care facility residents. Administration to health care personnel is based on the Advisory Committee on Immunization Practices (ACIP) December 1st meeting, of which recommendations were published online on December 3rd, and were subsequently adopted by the CDC Director.[i] This decision was based upon many factors, including the high-risk nature of exposures in healthcare settings, the burden of disease in health care personnel, predictive modeling demonstrating that more cases are prevented through vaccination of staff as compared to vaccination of residents in long-term care facilities, and the fact that vaccination of health care personnel is critical to preserving the capacity of the healthcare system to care for COVID and non-COVID patients.[ii] One of the key ethical principles that guided the Advisory Committee is that of maximizing benefits and minimizing harm. The ACIP workgroup consideration of ethical principles for vaccine allocation noted “the unanimity in opinion for early vaccination of health care personnel indicates that maintenance of health care capacity has emerged as a high priority in the context of a severe pandemic.”[iii] The guidelines of the Advisory Committee shed further light on the how this ethical tenet was considered, “these benefits include reduction of COVID-19-associated morbidity and mortality in HCP, which in turn reduces the burden on strained healthcare capacity and facilities; preservation of services essential for the COVID-19 response; and maintenance of the overall functioning of society…the ability of HCP to remain healthy helps to protect the health of others and minimize social and economic disruption.”ii
The Problem
While vaccinating health care personnel is critical to sustain the capacity of the healthcare system, which subsequently helps to avoid the disruption of healthcare services and limit social and economic consequences of the pandemic, a problem is presented by the vaccine itself. The vaccination, which represents the very answer to the pandemic for which the world has been waiting, does come with adverse reactions which themselves can magnify current problems with healthcare capacity. This issue is illustrated best by considering the adverse reactions extensively reported in the published report of the FDA on the Emergency Use Authorization for the Pfizer/BioNTech vaccine. It is noteworthy that systemic adverse events within 7 days after each of the two vaccinations included several symptoms and signs which mimic COVID-19 infection. Specifically, in recipients age 18 to 55, these adverse reactions included fever (7.5% of recipients), chills (28% of recipients), and myalgias (48.0% of recipients). While local reactions and several other systemic adverse events were also reported, these three symptoms are arguably the most similar findings to the disease itself. Data from the C4591001 Clinical Trial Group reinforce these concerns, and shed additional light on the nature and frequency of systemic adverse reactions. In that publication, some more granular data on systemic reactogenicity demonstrated that systemic adverse reactions, including fevers and chills, typically developed within 1-2 days of the vaccine and resolved within 2 days of onset.[iv] If health care personnel continue to be diligent with symptom self-assessments and health care systems continue to enforce procedures to keep personnel with these symptoms out of work, in order to protect patients and other personnel, this challenge represents a substantial problem which can further impair, in the short-term, the healthcare systems’ ability to maintain capacity. The fact that the vaccinations are beginning in the midst of a COVID-19 surge, when the healthcare system not only needs to sustain capacity, but in fact increase the capacity to care for patients, may represent a devastating challenge over the next several weeks.
Considering Solutions
When weighing how to approach this anticipated problem, it is important to recognize that the recommendations of the FDA Advisory Panel and CDC should not stop at determining the most appropriate allocation. While this was a monumental task, and one that is deeply important to society, it falls short. Equally important to determining what groups should be considered first for vaccine administration is the tactical approach to administering the vaccine. By providing guidelines and best practices to healthcare systems on how to approach vaccination among its critical personnel, the FDA and CDC could help limit the impact the vaccine has on decreasing critical staffing during an increasing COVID-19 surge.
The specific goal of such guidelines would be to avoid disproportionately affecting one group of personnel. For example, an obvious example arises when considering limited groups of personnel such as critical care nurses, respiratory therapists, and hospitalists. Without clear guidelines, if such personnel are left to just schedule vaccination themselves, this can result in a shortage of key health care workers when they are needed most. Recognizing that the vaccination can result in symptoms which mimic COVID-19 itself, one can assume, based on the above-noted adverse reactions, that 10% to 25% of personnel may be required to be out of work following one or both doses of the vaccine (25% is a low estimate based on the aggregate frequency of fevers, chills, and myalgias, but this percent can be much higher when considering the highest rates of those adverse reactions and other reactions not mentioned above, such as headache and GI symptoms, which may also warrant personnel to be out of work).
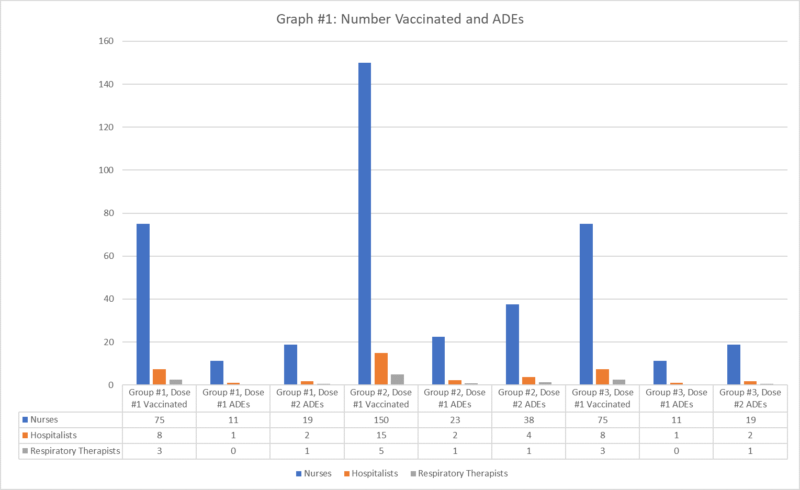
Accordingly, it is helpful to consider the impact of such adverse reactions on the healthcare workforce, and using an example is particularly illustrative. Assume, for the sake of this example, that General Hospital has 100 med-surg beds staffed by 300 nurses, 30 hospitalists, and 10 respiratory therapists, and that 25% of these personnel can be vaccinated in week #1, 50% in week #2, and 25% in week #3. The frequency of adverse reactions that would keep employees out of work, assuming a rate of 15% for dose #1 and 25% for dose #2, would suggest that 120 nurses, 12 hospitalists, and 4 respiratory therapists may be affected across the total 6 weeks of vaccination (Graph 1).
Recognizing that most systemic symptoms have onset and offset within ~3-4 days, if we assume that 50% of the time employees are scheduled to work while experiencing adverse systemic reactions and that each employee with systemic symptoms is out of work for 3 days, that represents shift deficits of 180 for nurses, 18 for hospitalists, and 5 for respiratory therapists across the 6 weeks (Graph 2). This is likely a low estimate given that during the current surge, many employees are working more than usual, so the rate that employees would be scheduled to work while experiencing adverse reactions would likely be higher than 50%.
If each of these groups of personnel are vaccinated randomly, such as through a self-scheduling process, this may result in higher or lower shift deficits, concentrated during certain periods of time. Without an organized process, this is, in fact, likely to happen. For example, if the hospitalists access the signup form earlier that nurses or respiratory therapists, due to aggressive notification within the group and administrative support for scheduling, that group’s shift deficit may occur during a single week, making shift deficits as high as 7 for the week that the first dose is administered and 12 following administration of the second dose. The same problem then persists, when the remaining dates are likely to include disproportionate numbers of nurses and respiratory therapists, now concentrating absences from those disciplines as well. The problem becomes magnified when considering that COVID case volume is projected to increase by over 20% during this same period of vaccination rollout.[v] This is likely to require additional staff in each area, accentuating the shift deficits. Such staffing shortages have major consequences for the staff that are left to compensate for staff that are unable to work and could impact access, quality, and safety of care as fatigue and staff-to-patient ratios decrease.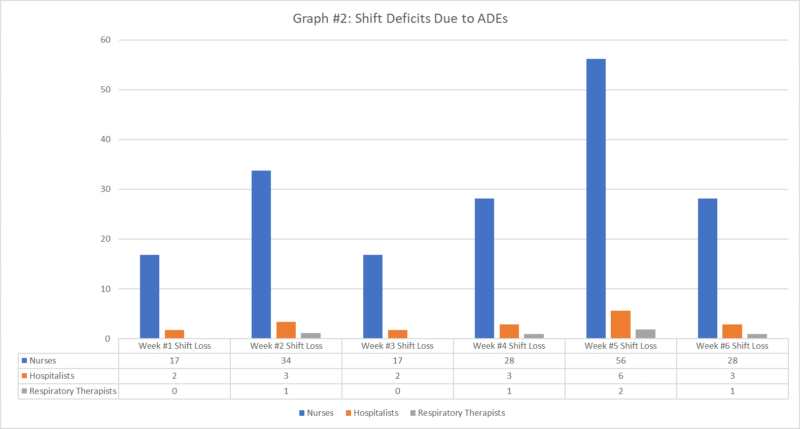
Proposing Guidelines
Vaccine provisioning at the level of health care personnel is complicated but can be optimized to limit the possible negative impact resulting from the initial rollout of the vaccination. While intelligent algorithms can likely be constructed to optimize vaccine scheduling to minimize staff absences due to adverse reactions, a manual process is quite manageable as well. If hospitals provide managers with vaccine administration schedules, the schedules can be cross-referenced with staff schedules to minimize times that vaccine administration would be immediately followed by work periods. Ideally, both vaccines would be scheduled such that personnel would have 3-4 days following administration where they are not scheduled to work. When vaccine administration schedules do not line up perfectly with a particular employee’s schedule, which will likely occur frequently, selecting vaccine administration dates that would correlate with the least number of shifts following the vaccine would be optimal. Allowing for conflicts following the first vaccine while prioritizing time off after dose #2, which has a substantially higher rate of systemic reactions, is another strategy that should be employed.
While employees will all be eager to receive a vaccine, it is important to remember the reason health care personnel are being vaccinated in the first place – to maximize benefits and minimize harm. Thus, it is at the population level that we have been selected to receive the vaccine, and it is likewise our responsibility to allocate it at the local level in a way that amplifies these same principles. Having a strategy to do so in a thoughtful, deliberate, and organized fashion will ensure that we all have the right place in the line to keep us safe and bolster our ability to care for the communities that we are committed to serve.
Guidelines to Consider for Vaccine Provisioning
- Avoid having staff individually schedule vaccinations; if staff prefer to schedule their own vaccination, consider pushing such requests to weeks later in the vaccination rollout.
- Develop vaccination schedules for health care personnel and provide these schedules to managers such that staff schedules can be coordinated optimally with vaccination dates.
- To optimize staff vaccination and date selection, keep the following in mind:
- Begin with education regarding the vaccination and then identify staff that plan to opt out of the vaccination in a non-judgmental, confidential manner.
- Make the work easy by leveraging spreadsheet tools to clearly display when staff’s schedules align with vaccination dates and when they have off for 3-4 days following vaccination dates. Working with an analyst, implement conditional formatting, for example, for this purpose.
- Work through each staff member’s schedule, identifying if there is an obvious option that optimizes vaccine administration.
- If there is more than one clear option, select the earlier date.
- If no choice allows for 3-4 days off following both doses #1 and #2, select the option that (a) decreases the conflicts during work periods following vaccine administration, and (b) prioritizes time off following dose #2.
- When a particular employee’s schedule does not allow for any option without many shift conflicts, consider selecting a dates for vaccination which are followed by shifts that are easiest to fill through moonlighting/per diem options.
- Following identification of best-fit vaccination dates, if the distribution of dates is not even and the hospital/health system are unable to support the number of staff that would be scheduled on a particular date, use a random process to review staff who would have been scheduled on that date, one at a time, to determine if another option would be a reasonable fit. Continue to work through employees on that date until enough staff have a different date identified.
- Managers, when considering their own vaccination, should either select a date later among the available options or delegate selection of their vaccination date to another person in their group.
- Managers should review the process with their staff and be transparent.
[i] Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Allocating Initial Supplies of COVID-19 Vaccine — United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1857-1859. DOI: http://dx.doi.org/10.15585/mmwr.mm6949e1
[ii] https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19/evidence-table.html, accessed 12/14/20
[iii] McClung N, Chamberland M, Kinlaw K, et al. The Advisory Committee on Immunization Practices’ Ethical Principles for Allocating Initial Supplies of COVID-19 Vaccine — United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1782-1786. DOI: http://dx.doi.org/10.15585/mmwr.mm6947e3
[iv] Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med 2020. doi:10.1056/NEJMoa2034577
[v] https://covid.cdc.gov/covid-data-tracker/#forecasting_weeklycases, accessed 12/15/20


Leave A Comment